Connecting Corals and Chemistry
September 1, 2009
Jay Lunden
Graduate Student
Temple University

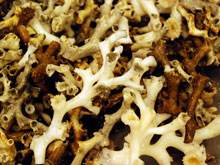
Lophelia pertusa on the seafloor. Note the extended polyp (on the right). Click image for larger view and image credit.
Cold-water stony corals, such as Lophelia pertusa, use a form of calcium carbonate called aragonite (think of a substance similar to chalk) to make their skeletons. To do this, the coral polyps must extract calcium and carbonate ions from the water. One of my primary goals on this cruise is to determine the availability of aragonite for corals in deep ocean waters. We call this availability the aragonite saturation state. I am excited about this research because no one currently knows the aragonite saturation state in the depths of the Gulf of Mexico. We are gathering the first data on the subject. In addition, rising levels of CO2 (carbon dioxide) in the atmosphere affect the aragonite saturation state in ocean waters, so the research is closely tied to global climate change.

Jay Lunden gets help from Lara Miles in collecting water samples from Niskin bottles mounted on the CTD rosette, which measures conductivity, temperature and depth (CTD). Click image for larger view and image credit.
Collecting Deep-sea Water
To measure the aragonite saturation state, we must first collect seawater where cold-water corals live. Oceanographers use a special instrument called a CTD rosette to collect seawater at different depths. The CTD is a conductivity-temperature-depth sensor, and the rosette has 12 water collection bottles (called Niskin bottles) arranged in a circle. The instrument is lowered to the bottom of the ocean and is then slowly brought back to the surface, stopping at pre-determined depths to sample water. When we bring the CTD back on board the ship, we have 12 water samples from varying depths. This water is retrieved and stored in the 5°C (41ºF) cold room until I am able to test each sample for alkalinity, a measure of the carbonate, bicarbonate, and hydroxide ions in the sea water.
Chemistry at Sea
To measure alkalinity, I am using a method called the Gran titration that assesses the buffering capacity of the seawater. First, I put a small amount of seawater in a beaker and measure the pH. Second, I slowly add sulfuric acid to lower the pH of the sample to 4.5. The greater the buffering capacity of the seawater sample, the more sulfuric acid required to bring the pH to 4.5. Once the pH reaches 4.5, I record the amount of sulfuric acid it took to get to that point and convert the number to units of alkalinity. Finally, I enter the alkalinity, pH, temperature, and salinity of the water sample into an equation to calculate the aragonite saturation state — and we have our data!
Interpreting the Data
Aragonite saturation levels are believed to change with depth, generally decreasing as we dive down to the seafloor. What do aragonite saturation state values mean? If the values are above a particular threshold, then the amount of carbonate, bicarbonate, and calcium ions are sufficient for stony corals like Lophelia to produce their skeletons. Low values indicate that it is more difficult to lay down the skeleton and grow. I hope to be able to identify the threshold in the aragonite saturation state values where enough aragonite is available that Lophelia colonies can thrive in deep, cold waters. Understanding this threshold will be important in helping us predict more accurately the distribution of Lophelia on the ocean bottom, and the future impacts of ocean acidification.