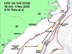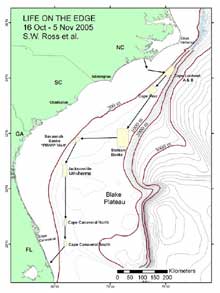
Figure 1. Map of the southeastern US study area, illustrating proposed path of the cruise and various deep coral sampling areas. Click image for larger view and image credit.
View a slide show of a variety of types of coral habitats. The researchers will examine the relationships of different morphologies of corals to their environments and their genetic structures.
![]() Click
image to view a slide show.
Click
image to view a slide show.
Mission Plan
Steve W. Ross,
Chief Scientist/Lead PI
Center for Marine Science, University of North Carolina at Wilmington and
US Geological Survey
Our research on selected habitats (outer shelf reefs, canyon systems, deep-sea corals) in the southeastern US and Gulf of Mexico involves a number of co-investigators from multiple agencies. Currently, in addition to Lead PI Ross, co-principal investigators include Dr. K.J. Sulak (USGS), Dr. M.S. Nizinski (NOAA Fisheries), and E. Baird (NC Museum Natural Sciences). Additional collaborators include Dr. C. Morrison (USGS), Dr. M. Risk and B. Williams (McMaster Univ.), A. Howard (ARTWORK), and Dr. C. Holmes (USGS). A deep coral scientist from the European community, Dr. J.M. Roberts (Scottish Association for Marine Science), will also join our expedition this year. See Explorers section for more details and other critical members of our team.
Deep coral habitats are more extensive and important than previously known, and at the same time they are being threatened. These high profile features may concentrate exploitable resources and enhance local productivity in ways similar to seamounts, but this has not yet been explored. Even though hypotheses have been posed on how coral banks form, data are lacking. Live coral ages and growth rates are poorly known in this area, and ages of coral mounds or dead coral stands are also limited or equivocal. The degree to which there is an obligate deep coral fauna is unclear. While the genetic structure of Lophelia in the northeastern Atlantic is being described, such studies are just beginning in the western Atlantic. It is for these reasons that locating, describing, and mapping deep corals and conducting basic biological studies in these habitats are priorities for our research. On our previous cruises, we have documented deep coral and outer shelf reef habitats and associated biota. However, these missions have only begun to quantify this extensive habitat off the southeastern US (SEUS). Our studies have also yielded many new records of biota, new ecological data, new data on habitat distributions/structures, and data on water column trophic connectivity. This cruise continues and expands our explorations of these unique and relatively unknown deep-water habitats.
We will locate and sample poorly studied middle slope coral banks (Lophelia mostly, 360-800 m) from Cape Lookout, NC to southeastern FL (see map Figure 1). We will use a manned submersible (Johnson Sea-Link, JSL) to quantify biota and habitat on and near reefs via standardized video transects and point observations. The JSL will collect samples of corals and associated invertebrates for various purposes. Each dive will probably have multiple objectives, with some tasks given priority. We will also sample the whole water column using a variety of ship deployed nets.
During this expedition, we will cover over 650 nautical miles (1,205 km). We will try to conduct all long transits between major dive areas at night in order to maximize daytime submersible usage. This will reduce our night sampling activities with the exception of continuous sonar surveys of the sea floor, which will be conducted during these night transits. First, the SEWARD JOHNSON will steam for coral banks located off Cape Lookout, NC, where we will work 1-2 days (17-18 Oct) depending on the quality of the sites and objectives. We will move south overnight to the Cape Fear coral bank, working there for 2 days (19-20 Oct). Next, the ship will move (again overnight) toward the “Stetson” coral bank area, east of Charleston, where we will probably work for 3-4 days (21-24 Oct). On the way to that site, we will try to conduct a brief bathymetric survey of suspected coral areas just south of Cape Fear. If mounds are located, we may adjust the schedule to dive on them. After the Stetson area, we will steam westward toward the Savannah coral banks, where we will work for about 2-3 days (25-27 Oct). While at this site (25 Oct), we plan to rendezvous with a vessel coming out of Charleston which will bring replacement personnel, media personnel, and VIPs to view our work. We will give a tour of the vessel and explain our research. The next work sites will be the large area that starts off Brunswick, GA-Jacksonville, FL. We may be in this area 3-4 days (28-31 Oct), working several sites, as we head south. The last areas of work will be from just north to just south of Cape Canaveral, FL, where we will conduct dives for 3-4 days (1-3 Nov). Considering the time of year, we will be prepared for marginal weather and will plan other activities if the submersible cannot be used. At the end of sampling (evening of 3 Nov) the ship will head for port in Ft. Pierce to arrive around noon (tide-dependent) on 4 Nov. Our crews will unload and depart as soon as possible.
1) Locate and document areas of known and suspected coral concentrations
between Cape Lookout, NC and southeastern Florida
2) Continue to define benthic fish and invertebrate community structures
3) Classify reef and off-reef habitat zones and document faunal affinities
4) Explore habitat affinity of deep reef fishes and macroinvertebrates
5) Age deep-water corals (mostly Lophelia)
6) Retrieve long proxy temperature and productivity records deep corals
7) Collect Lophelia, other corals, and associated fauna and amplify and
sequence their DNA for phylogenetic, phylogeographic, and community genetics.
Determine degree of relatedness of Lophelia coral banks in the SEUS using
microsatellite markers and relate this information to reef topology, other
abiotic characteristics, and to coral morphology.
Education/Outreach Objectives:
1) Provide at sea education experience and real-time communication to
the public
2) Provide education/outreach products to a variety of audiences
3) Provide at sea science research experience for an educator
4) Develop an interactive webquest to complement cruise objectives
5) Add to High Definition video and digital still images for education
and outreach projects.
Sampling Methods
Although we plan to be as flexible as possible to respond to unforeseen events, most submersible dives follow a similar pattern, emphasizing bottom transecting, collecting and photographing specimens. We plan to descend through the water column quickly. During descent we will take notes/observations on distributions/behaviors of fauna. Any time that “swarms” of mesopelagic nekton are around the submersible, we may try to collect (& film) these. Specimen collecting (using submersible suction device into sampling canisters) may begin as soon as possible after landing on the bottom. After some collecting time, the video camera will be moved to pre-determined pan/tilt positions, set on wide angle, and the submersible will proceed to run video transects. Transects will be run across all habitat types, including both coral and non-coral areas, at very slow speeds with the JSL as near to bottom as possible. Transects should take place on every dive; however, due to time constraints other activities may be allocated to certain dives only. Additional specimen collecting may occur after these transects. Still photography (using digital cameras) and videography will be conducted throughout the dive (not just during transects). At several locations and depths (not necessarily every dive) corals, fishes, and benthic invertebrates will be collected for genetics and other analyses. Traps may be deployed during some dives and may be left on site until later dives retrieve them. Scientists in the bow and stern compartments will have digital audio recorders, digital video cameras and still cameras for use within the sub.
Additional samples will be collected by gear deployed from the ship when the submersible is not being used. Bottom trawling with small otter trawls (30-minute tows) will be emphasized in the depth range of about 300 to 700 m in areas where there are no corals. We will also deploy surface nets (3m neuston and 1 m plankton) for 15-minute tows. As time or circumstances allow, night lighting (using dipnets, cast nets, etc.) from the side of the ship will be conducted during night watches with the ship slowly drifting.

Figure 4. (Top Image) Bringing in the Tucker trawl, a large net used to sample mid-water depths. It can be triggered to open or close, thus giving a depth discrete sample. (Bottom Image) Tucker trawl catch of small red krill, long slender snipe eels, sergestid shrimp and lanternfish. Click image for larger view and image credit.
Although substantial data (identifications, numbers, sizes, etc.) will be taken at the lab on shore after the cruise, some specimen work up will occur at sea in the ship’s labs. Many of the fishes and invertebrates caught on this cruise will be small and will be preserved onboard in 10% formalin soon after capture. Many invertebrates will be preserved in alcohol. Many corals will be dried. For species that are easy to identify or too large to save, we may dissect stomachs from the specimens and take length, sex, and other data at sea. Subsamples of selected species (we have a target species list) from various habitats may be selected for stable isotope analysis. A section of white muscle from these will be dissected soon after capture, dried, and stored so that they are ready for further analysis. Subsamples (fresh) of selected species will be set aside for on board photography. Our data crew will be organizing and digitizing data collected by each watch as in past cruises. We plan to have a digital database of the station data for the PIs by the end of the cruise. There is a large amount of data coming from multiple instruments (see diagram Figure 6), collected for multiple purposes. Data organization and tracking are very important.
For more information
www.lophelia.org ![]()
Lophelia.org is an information resource on the cold-water
coral ecosystems of the deep ocean.