 |
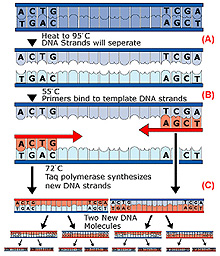
Polymerase chain reaction (PCR). Step1: Solution
is heated to 95°C to denature (unzip) the two strands of the
target DNA (A). Step 2: Solution is cooled to ~55°C
to allow the primers to anneal (bind)
to the ends of the DNA strands (B). Step 3: Solution
is reheated to ~75°C to allow
TAQ polymerase to synthesize complementary
copies of each strand. Click image for larger view.
|
|
How Do You Perform DNA Analysis of Corals?
Marion
Beal
Geneticist
SCDNR Genetics Laboratory
Hollings Marine Lab
Analysis of DNA isolated from deepwater corals can provide information
on the relationships among coral colonies of the same species, and the
evolutionary relationship among different species of corals. Genetic
analysis can also tell us how corals reproduce (sexual reproduction vs.
colony fragmentation and growth of fragments). This analysis can
help us understand how corals are dispersed to new habitats (like seamounts)
as they are formed, or how corals might recruit to an area where they
have previously been eliminated by natural or man-made destruction.
There are a variety of techniques used to analyze DNA. Because
very little is known about the life history of deep-sea coral species,
population genetics is a very useful tool for gaining knowledge on reproduction
and dispersal. The relatively sedentary life of adult coral, and
reproduction of colonies by fragmentation can lead to populations or
groups of colonies that are genetically isolated from other populations. However,
sexual reproduction by broadcast spawning and dispersal of tiny free-swimming
larvae provide mechanisms of gene flow for corals that may result in
geographically distant populations being similar in genetic structure. Variability
in the sequence of base pairs in the DNA molecule allows us to identify
genetic differences that may accumulate between individuals and populations,
and to define the historical or evolutionary relationships based on these
traits. Genetic data may also be used to help understand biological,
oceanographic and geographic factors that isolate populations and lead
to genetic and morphological differentiation.
 |
Agarose gel electrophoresis
of PCR product. Click image for larger
view.
|
|
Collection and storage of DNA has become relatively simple, as new technology
allows us to obtain analyzable DNA from only a small
tissue sample that can be preserved using simple methods. Small
tissue samples can be placed in preservative solutions
and stored at ambient temperature, and the DNA in the tissue will remain
stable for several years. After
isolation from the tissue, the DNA can be further stabilized
and stored dry.
As the first step in the analysis, DNA must be isolated from the other
organic materials in the tissue sample. Isolation methods take
advantage of the differences in molecular size and structure between
DNA and proteins, lipids and other large molecules in the cells from
which the DNA is extracted. Most analysis techniques require very
small amounts of DNA and one sample can be used for several different
analyses. Microsatellite and mitochondrial DNA (mtDNA) are commonly
used for analysis because they are mutate rapidly and are highly variable
regions of the DNA. In addition, variation in the sequence of bases
in the DNA and the resulting allelic variations are easily revealed with
fairly simple molecular techniques.
DNA can replicate itself in a cell, and geneticists use this characteristic
to produce many copies of the DNA in laboratory reactions from a small
tissue sample. The polymerase chain reaction (PCR) is used to produce
millions of copies of a specific section of DNA that has been isolated
from the tissue sample. Amplification takes place in a matter of
several hours. During that time, a reaction containing a pair of
short priming DNA sequences complementary to the ends of the target sequence,
TAQ DNA polymerase, and a solution of deoxynucleotides (dNTPs) is repeatedly
heated and cooled to denature the double-stranded DNA, synthesize a new
strand to complement each of the denatured strands, and to repeat the
cycle. In order to visualize the DNA, amplified PCR product is
loaded into a gel matrix. When an electrical current is applied
to the gel, the DNA migrates through the gel at a rate that depends on
fragment size. After the run, DNA trapped in the gel can be visualized
using the chemical ethidium bromide, which integrates into the DNA, and
fluoresces under ultraviolet light.
 |
Examining an image of a gel
for the presence of microsatellite DNA, indicated
by the repeating pattern of bands. Click image for larger view.
|
|
After confirming the presence of amplified DNA of a size that agrees
with the size of the sequence targeted for amplification, the amplified
mtDNA can be digested with restriction enzymes. Restriction enzymes
recognize certain sequences of DNA and cut the DNA into pieces at those
sequences. A population that possesses a mutation in the DNA, so
that it does not have that particular sequence, will end up with DNA
fragments after the digestion that are different from other populations. When
the amplified DNA that has been subject to digestion reactions is subjected
to gel electrophoresis, smaller fragments of DNA travel farther in the
gel than larger fragment, thus revealing variations in the sequence of
base pairs in the amplified DNA. It also reveals differences that
may reflect species or population differences among the samples of coral
from which the DNA was obtained. Microsatellite DNA amplified by
PCR is not subjected to restriction digestion, but may differ among populations
in the size of the microsatellite. Again, because of different
sizes of the DNA molecule in different-sized microsatellites, the microsatellite's
DNA will migrate through the gel at different rates, based on size of
the sequence amplified. Microsatellite size varies among populations,
and the resulting gel can reveal population differences based on the
frequency of microsatellites of differing sizes.
For an even closer examination of variations, variable regions within
the mtDNA or microsatellite DNA can be sequenced from PCR products revealing
single nucleotide differences between individual sequences.
 |
Loading DNA sample into a
gel. The gel will be subjected to an
electric current, which will cause the DNA
molecules to move through the gel. The
distance and speed they move is related to
the size of the DNA fragments, which varies
from species to species or among populations. Click image for larger view.
|
|
The data on the frequency of detected sequence differences among individual
samples (coral colonies) can then be statistically
tested with software designed for molecular genetic
analysis. Large
numbers of alleles are indicative of high genetic variability
in a population and large numbers of shared alleles among populations
is evidence for gamete or larval exchange between communities. On
the other hand, small numbers of alleles indicate low genetic variability
or possible “inbreeding” and
low number of shared alleles between populations may
be due to poor dispersal of larvae or gametes.
DNA samples collected from
corals or other organisms can be archived and stored
for future research, and samples can be easily exchanged among investigators. Molecular technologies are advancing
rapidly and leading to new ways to analyze DNA. In the near future
there could be endless possibilities for analysis of
samples collected today.
Fred Andrus
Department of Geological Sciences
University of Alabama
Analysis of deep-water corals is significantly different from analysis of other corals, not only because deep-water corals grow in unique and hard to reach environments, but also because the average size of the samples is so small. Unlike massive corals such as those found on tropical reefs, deep-sea corals of the Blake Plateau are often small solitary corals or small and delicate colonies. When the samples collected during the Estuaries to the Abyss expedition are returned to the laboratory, our analyses will include determining the elemental and isotopic chemistry of the coral skeleton. Unlike vertebrates, which generally dissolve and replace their skeleton as they grow, corals add new material on top of the old, thus preserving a record of its developmental history. This material is composed of calcium carbonate in the form of aragonite.. The chemistry within this accretionary skeleton is often a reflection of the coral's environment, and therefore an old coral contains a record of changes in the conditions around it for many years as it grew.
Most climatological and ecological reconstruction research using shallow-water corals focuses on massive colonial species that are relatively fast-growing and may live for many decades. In contrast, most deep-water corals appear to be slower growing and are often much smaller, whether they are colonial or solitary. To measure detailed chemical variation in the life history of a coral, including changes that occurred at monthly or even shorter time intervals, we must make many measurements within a relatively small area of the coral skeleton. This is difficult to do with the smaller deep-water corals. Whereas shallow corals may grow several millimeters a year, it is common for deep-water corals to grow at less than one millimeter a year. This means that the many tiny samples are required in these delicate skeletons to study time-related effects and characteristics.
We perform these analyses by isolating a small portion of each coral and measuring the chemistry within just a small region. Many samples can be measured in a line starting at the oldest part of the skeleton and moving towards the areas grown just prior to collection of the specimen. This creates a profile of change over time. This may entail the use of in situ (in place) techniques where an intact slice of the coral is placed in the path of a beam of electrons. The way these electrons interact with the sample surface indicates what major elements are present, creating a "map" of the chemistry of the coral. Very little damage is done to the coral sample during this procedure.
If we need to identify and measure the amounts of rarer chemical elements, we may have to sacrifice more of the coral sample using a different technique. A narrow laser beam can be used to ablate or burn off minute pieces of coral, creating small pits (fractions of a millimeter) in a slice of the coral. The gasses released from the ablation of the material are then analyzed to determine what elements made up that portion of the coral. Although more destructive to the specimen, this technique produces very useful data from areas that are very small, so that many small samples can be obtained from a single coral slice.
In order to measure isotopes within a coral, a larger sample is needed. Isotopes are atoms of an element that contain different numbers of neutrons. The relative amounts of different isotopes of the same element can tell us something about the environment that the coral grew in (for example, water temperature). Rather than taking the measurement in place, we need to physically remove a portion of the sample and then analyze it later by one of several techniques. Our approach has been to mill out samples using a drill, similar to that which a dentist uses, but rather than being held by hand, a computer-driven machine is used. We can use this tool to “whittle” away small samples (often less than one tenth of a milligram). Each is then measured for isotopes.
Deep-water coral analysis requires special tools and techniques, from the complex task of collecting the samples with a research submersible or Remotely Operated Vehicle to the detailed and delicate sampling required to measure the chemistry of their small and fragile skeletons. The work that is conducted on the ship during the expedition is just the beginning of a long process that can take years to complete.
|

