Coral Age Dating
The passage of time can be measured in many ways. For humans, the steady movement of the hands on a clock marks off the seconds and the hours. In nature, the constant decay of radioactive isotopes records the march of years. Scientists can use the clocklike behavior of these isotopes to determine the age of rocks, fossils, and even some long-lived organisms.
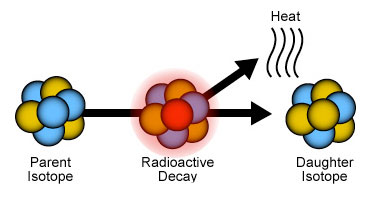
Isotopes are forms of an element that have the same number of electrons and protons but different numbers of neutrons. Some of these atomic arrangements are stable, and some are not. The unstable isotopes change over time into more stable isotopes, in a process called radioactive decay. The original unstable isotope is called the parent isotope, and the more stable form is called the daughter isotope.
Isotopes decay at an exponential rate that that can be described in terms of half-life. One half-life is the time it takes for ½ of the parent isotopes present in a rock or bone or shell to decay to daughter isotopes. Parent isotopes decay to daughter isotopes at a steady, exponential rate that is constant for each pair.
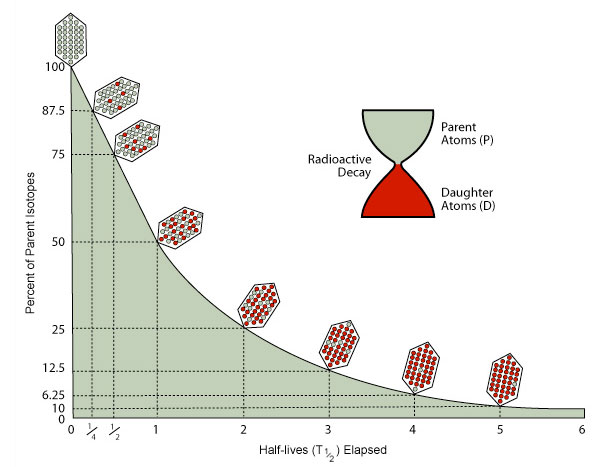
The shape of this curve is the same for the radioactive decay of all isotopes. The amount of actual time in a half-life is unique to each parent/daughter pair, however.
In this lab, you will use radiometric dating techniques to calculate the ages of living and dead corals on a seamount. You will then use this information to determine environmental conditions on the seamount.
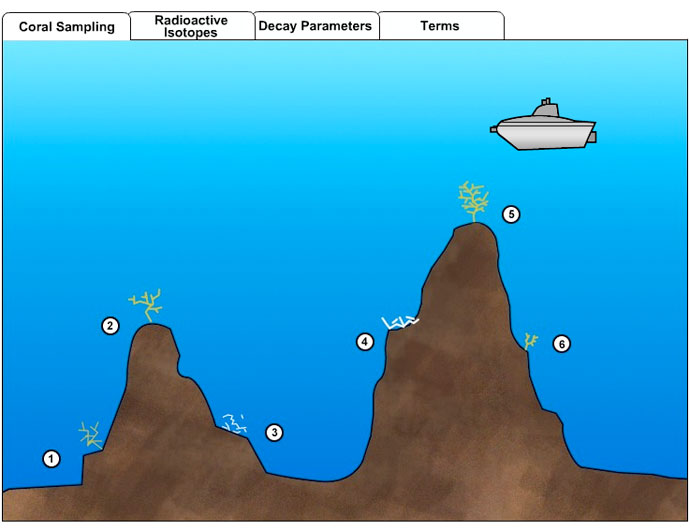
Instructions:You are a marine scientist studying the deep-sea corals growing on a seamount. In order to understand coral life and history, you need to know something about the age and growth patterns of these organisms. One way to do this is with radiometric dating. As a coral animal grows, it secretes a hard external skeleton. Radioactive isotopes absorbed from seawater by the animal are incorporated into the skeleton, where they begin to undergo radioactive decay. Radiometric dating will reveal the age of individual corals on the seamount.
Use your submersible to travel about the seamount and drill samples from the skeletons of living and dead corals. Then calculate either the percentages or ratio of parent to daughter isotopes from the isotopic data measured in each sample. Use the decay parameters chart to translate those figures into the number of half-lives that have elapsed. Multiply that by the length of a half-life for that isotope page to determine the age of the coral.
Questions
- Describe how radioactive decay can be used to date rocks and organic material.
[Check Answer]
Radioactive isotopes break down over time, changing from parent isotopes to daughter isotopes at a steady rate. When a rock or shell or bone is formed, 100% of the isotopes in it will be parent isotopes. As time passes, the percentage of parent isotopes drops and the percentage of daughter isotopes rises. The ratio of parent and daughter forms reveals how much time has elapsed.
- Calculate the ages of the six coral samples.
[Check Answer]
Coral 1: Parent isotope (Pb 210) to daughter isotope (Pb 206) ratio = 25/375 = 0.067. According to the Decay Parameters Chart, that P/D ratio means 4 half-lives have elapsed. Since 1 half-life of Pb-210 is 22.26 years, the coral is 4 times that old, or 89 years.
Coral 2: The sample contains 13% Pb 206, which is the daughter isotope. Thus it must be 87% parent isotope, for a P/D ratio of 7, which means ¼ of a half-life has gone by since that part of the coral skeleton formed. The coral is 22.26/4, or 5½ years old.
Coral 3: Parent isotope (C 14) to daughter isotope (N 14) ratio = 525/3500 = 0.15, or about 3 half-lives. One half-life of C-14 is 5,730 years, so the coral grew 17,190 years ago.
Coral 4: % of parent isotope (C-14) = 75% = ½ of a half-life. Half of 5,730 is 2,865 years.
Coral 5: The ratio of parent to daughter isotopes is 1, so 1 half-life has elapsed. The coral is 22 years old.
Coral 6: P/D = 300/100 = 3. That is equivalent to 0.5 half-lives, or 11 years.
- Based on these dates, which way does the current flow past these seamounts?
[Check Answer]
The dead coral on the seamount on the left is considerably older than any of the corals sampled on the other seamount. Based on that, it seems likely that the left seamount was colonized first, then currents moving left to right across the image carried coral larvae over to the second seamount.
- Compare the sizes and ages of the four living corals. Can you make any conclusions about where the most favorable living conditions are located?
[Check Answer]
The corals growing on the summits have faster growth rates. They are as larger or larger than older specimens found on the flanks of the seamounts. Perhaps current patterns concentrate food on the peaks or sweep away mud or predators that inhibit rapid growth.
- Why were some corals dated using Carbon-14, and others with Lead 210? Why can't Lead 210 - Lead 206 dating be used for anything more than about 150 to 200 years old? (HINT - look at how the slope of the decay curve changes over time)
[Check Answer]
Radiometric dating will be very hard to do if only a small fraction of a half-life has elapsed. Early on the amount of daughter isotopes present will be very small, and difficult to measure accurately. On the other hand, after several half-lives have gone by, the actual number of parent and daughter isotopes will change only slightly over long periods of time, so accurate age calculations will be very difficult.
So Lead 210 dating worked well for the living corals, but was inappropriate for the much older dead corals. Carbon-14 dating would not have been useful for corals only a few years old, but does work well for organic material that is thousands of years old.
Related Links
Multimedia Discovery Missions: Lesson 15 - Seamounts