
By Robert Cowen, Ph.D., Director and Project Lead - Hatfield Marine Science Center, Oregon State University
R/V F.G. Walton Smith: August 14-28, 2014
M/V Spree: June 18-27, 2014
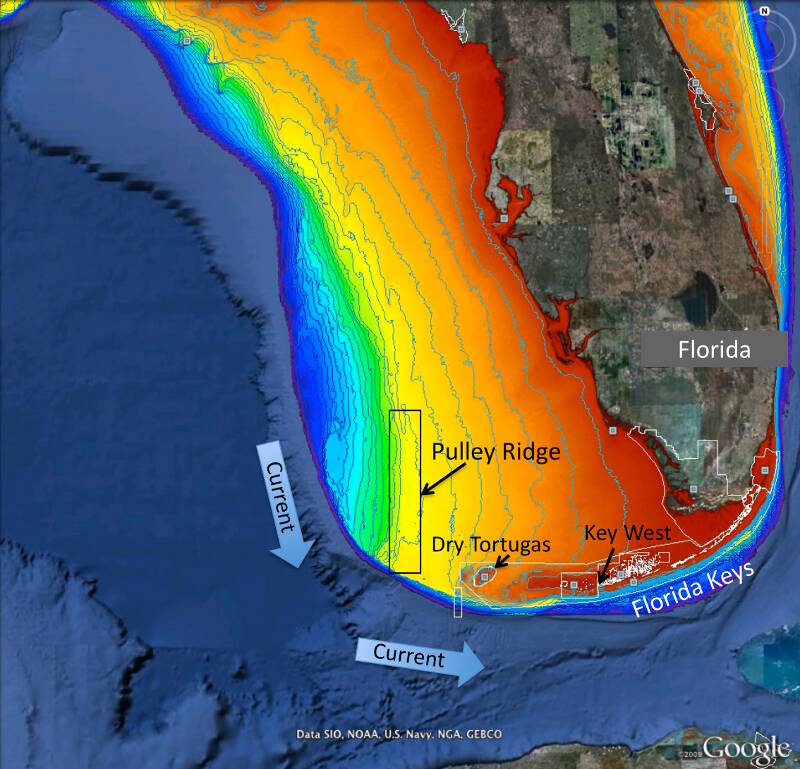
Figure 1. Map of project area showing Pulley Ridge, off the west coast of Florida at depths of 200-330 feet in relation to the downstream refs of the Dry Tortugas and Florida Keys. Colors represent water depth, which ranges from 33 feet (red) to depths of 820 feet or greater (dark blue). Current arrows depict prevalent current direction. Background image is from Google Earth and the depth information is from the U.S. Geological Survey and NOAA. Image courtesy of Robert Cowen. Download larger version (jpg, 1.4 MB).
This is the third year of a five-year study to investigate the role that the mesophotic reefs of Pulley Ridge (off the southwest coast of Florida) may play in replenishing key fish species, such as grouper and snapper, and other organisms in the downstream reefs of the Florida Keys and Dry Tortugas (Figure 1).
Mesophotic reefs are coral reef environments found at depths ranging from 30-40 meters to greater than 100 meters in the Gulf of Mexico where sufficient light enables certain reef-building corals (i.e., corals with symbiotic algae growing in them) to survive. Mesophotic reefs support a diversity of populations of algae, sponges, corals, other invertebrates, and fishes.

Figure 2. In 2014, we will be using a new remotely operated vehicle, the SubAtlantic Mohawk 18, owned by the National Marine Sanctuary Foundation and the Flower Garden Banks and operated by the Undersea Vehicles Program at the University of North Carolina Wilmington (UVP/UNCW). Image courtesy of UVP/UNCW. Download larger version (jpg, 1.9 MB).
In the summer of 2014, we are conducting our third year of fieldwork, using two separate vessels. The first cruise took place on the M/V Spree (a charter dive vessel operated out of Key West, FL) from June 18-27; read a summary here. The second cruise will occur on the R/V F.G. Walton Smith (owned and operated by the University of Miami/RSMAS) from August 14-28 (Figure 2). A total of seven science divers and one technician were on the Spree, while 12 scientists and technicians will be on the Walton Smith. Each vessel will address a different facet of the project.
On the Spree, the science divers focused on collecting specimens of target taxa for population genetic analyses in the laboratory. We made these collections in areas that have been shown to support higher-than-average densities of the target species.
All specimens will be used for genetic studies and age and growth information for the fish species. Additionally, the divers recovered and redeployed instruments that track oceanographic currents during this mission.
On the Walton Smith, the science team will focus on:

Figure 3. A larval squirrelfish of the Family Holocentridae. Larval fish are sampled using plankton nets or light traps. Image courtesy of Cedric Guigand. Download larger version (jpg, 359 KB).