Gernot Friederich is busy preparing the mass spectrometer for its first deployment. Click image for larger view and image credit.
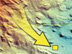
Unlike most of the science instruments (which are loaded onto the front of the remotely operated vehicle [ROV]), the mass spectrometer is bolted down on the back of the ROV Jason. Here we see the location of the "mass spec" (indicated by the red arrow) on the Jason. A probe, connected to the body of the instrument, is located on the front of the Jason and is used for measuring dissolved gases. Click image for larger view and image credit.
"Sniffing" for Chemicals at 13,123-foot Depth
June 23, 2007
Peter Girguis
Harvard University
27° 6.66 N
91° 9.91 W
![]() Deployment of the mass spectrometer. (Quicktime, 1.8 Mb.)
Deployment of the mass spectrometer. (Quicktime, 1.8 Mb.)
![]() Mass spectrometer schematic. (Quicktime, 496 Kb.)
Mass spectrometer schematic. (Quicktime, 496 Kb.)
During this research cruise, we will be using the mass spectrometer to measure dissolved gases around hydrocarbon seeps. We think the distribution of hydrocarbon gases — such as methane, ethane, butane, and propane, as well as hydrogen sulfide — plays a key role in determining which animal communities inhabit these environments. The underwater mass spectrometer, which we call the in situ mass spectrometer (ISMass), will be used to construct a geochemical map of key dissolved gases in conjunction with the other ecological data being collected by the other researchers.
Mass spectrometers are machines that separate chemicals from one another by their size and their charge. Because of their flexibility and high sensitivity, mass spectrometers have been very useful in research. In particular, quadrupole mass spectrometers are instruments that can measure concentrations (and partial pressures) of a wide range of gasses. These mass spectrometers can detect a broad range of compounds — from hydrogen to heavy hydrocarbons — with a single detector, with high sensitivity and rapid analysis.
As is often true, there are obstacles to overcome when developing instruments. In this case, mass spectrometers must operate in a vacuum in order to ionize (or charge) the gases being introduced into the instrument for analysis. While this would not be a problem in outer space (which is the only place where a high vacuum naturally exists), it is a big problem in the deep sea, where pressures are very high. Specifically, our mass spectrometer must be able to remove dissolved gases from seawater at pressures 450 times greater than the atmosphere (or 6,000 pounds per square inch) while maintaining a high vacuum on the inside of the analytical chamber. That was our biggest challenge: to find a way of sampling gases from water at high pressure and then analyzing them in a high vacuum.
Before ROV deployment, Peter Girgius bolts the mass spectrometer to the Jason. Click image for larger view and image credit.
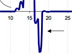
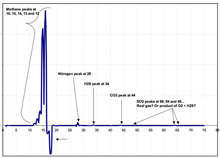
An example plot of the real-time data streaming in from the in situ mass spectrometer. Scientists will use this data to better understand the role that hydrocarbon gases — such as methane, ethane, butane, and propane, as well as hydrogen sulfide — play in determining which animal communities inhabit seep areas. Click image for larger view and image credit.
As we quickly found out, there are no companies that make a sampling system that would suit our purposes. As such, my colleagues (Jon Erickson, Gernot Freiderich, and Scott Wankel) and I decided to build our own sampling system. We followed the example of researchers using membranes to sample gases at atmospheric pressure (1 atmosphere). This is achieved by allowing the sample air to pass across a gas-permeable membrane. A vacuum pump on the inside of the mass spectrometer pulls dissolved gases out of the seawater and into the mass spectrometer for analysis. In the deep-sea, we use this membrane to keep extremely high-pressure water out while allowing gases to freely move through and into the mass spectrometer. This 25-micron (micrometer) thin membrane serves as the only piece separating the extremely high pressure of the sea floor from a vacuum similar to the one found in outer space. As it turns out, our membrane inlet system does just that. It can accommodate these very high pressures, allowing us to use this mass spectrometer directly in deep-sea environments. Quite an amazing feat!
So far, we have had two very successful deployments and have measured the dissolved gases from around bacterial mats and sediments. We were able to see low concentrations of methane around the bacterial mats and much higher concentrations of methane in the sediment beneath it. This is the first time that we've been able to measure dissolved gases at Gulf of Mexico seeps in real time. We were sitting on the ship controlling the instrument’s sampling patterns and data analysis. That was an amazing moment!