Methane Hydrates - Is it hot in here, or is it just me?
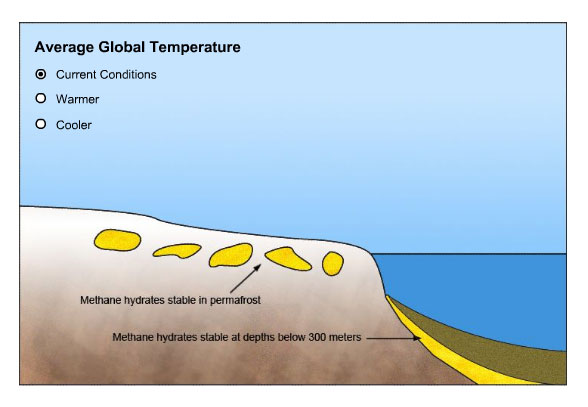
Methane hydrates are a frozen amalgam of natural gas and water. Made of molecules of methane (CH4) enclosed in a cage of ice, hydrates are squeezed together by high pressure and low temperature. They form in two settings - in polar permafrost where the ground temperature stays below 0º C, and in seafloor sediments deeper than 300m.
Methane hydrates are being actively investigated as a potentially enormous energy source. Scientists estimate that hydrates contain twice as much carbon as the combined global reserves of oil, coal, and other types of natural gas.
They have also been proposed as major agents of climate change. Methane is a powerful greenhouse gas, ten times more effective at trapping heat than carbon dioxide. But the volume of this gas now in the atmosphere pales next to that currently sequestered in hydrates, estimated at ten thousand billion tons (about 3,000 times the amount of methane as the atmosphere).
In this activity you will investigate the conditions that affect the stability of methane hydrates, and lead to the release or removal of methane gas from the atmosphere.
Instructions: Study the current distribution of methane hydrates carefully. Then click the image to change the average global temperature and notice how hydrate deposits react to cooler and warmer conditions. Once you've studied all three situations, answer the questions that follow.
Questions
- Where are methane hydrates now found?
[Check Answer]
There are methane hydrates within the permafrost in Arctic areas, where cold temperatures year round keep them frozen. The sediments of the deeper parts of the continental shelves also contain methane hydrates, where they are stable under the cold, high-pressure conditions found there.
- Describe the impact of an increase in average global temperature on sea level and on marine and terrestrial hydrates. Explain any changes.
[Check Answer]
When average temperature rises significantly, sea level goes up because glacial ice and snow melts, and because warm water expands. As the oceans rise, they flood low-lying lands. Even in high latitudes, this water is above freezing, and any permafrost it covers will start to thaw. As the ground warms up, methane hydrates within it will break down - the ice will melt, allowing the trapped gas to escape into the atmosphere. Marine hydrates will remain stable, because rising sea level will only increase the pressure on them.
- How would the release of methane into the atmosphere affect rising temperatures?
[Check Answer]
Methane is a greenhouse gas, so it traps heat in the atmosphere. If the amount of methane in the atmosphere increases, the temperature will go up even more.
- Describe the impact of a decrease in average global temperature on sea level and on marine and terrestrial hydrates. Explain any changes.
[Check Answer]
When temperature drops, so does sea level, as more and more water is locked into ice and snow on the continents. Falling sea level will reduce the water pressure on ocean floor deposits, so hydrates will start to break down, beginning in the shallowest areas. Methane gas will bubble up from the bottom, rise through the sea, and eventually diffuse into the air. Terrestrial hydrates are not affected, because colder air preserves the permafrost that protects them.
- How would the release of methane into the atmosphere affect falling temperatures?
[Check Answer]
Because methane is a greenhouse gas and so tends to raise temperatures when it builds up in the atmosphere, the trend to colder conditions will probably slow down or even reverse.
- Speculate on the role the formation and dissolution of methane hydrates may have played in the waxing and waning of the Ice Ages, specifically the slow advance then rapid retreat of continental glaciers.
[Check Answer]
The feedback between global temperatures and methane hydrates could help explain some of the patterns that occur when the climate flips between glacial and interglacial stages. As glaciers slowly advance, sea levels will slowly drop, and methane will start to accumulate in the air. Perhaps there is a "tipping point" or a level at which the quantity of atmospheric methane is sufficient to overpower the cause of falling temperatures and cause sudden warming.
- What impact could methane hydrates play in the course of global warming?
[Check Answer]
If the climate warms enough to lead to a significant melting of permafrost, large amounts of methane will be released into the atmosphere. A methane build-up will greatly enhance the intensified greenhouse effect that is driving global warming, and could cause the temperature to rise even higher, and to rise quickly.
Related Links
Multimedia Discovery Missions: Lesson 11 - Energy from the Oceans